Write the Isotopes of Hydrogen Explain Their Differences
There are two methods ways for specifying identifying isotopes. Deuterium hydrogen-2 is the second most abundant isotope of hydrogen and it makes up 00026 to 00184 of the hydrogen that is naturally found on the Earth.
Isotopes Of Hydrogen Plutonium Deuterium Tritium With Examples Videos
The atomic number is defined by the number of protons present in the atom.

. _ 1 3textrm H 1. Atomic weight of hydrogen is 1007947 whereas the mass of deuterium is 2014102. An isotope is named after the element and the mass number of its atoms.
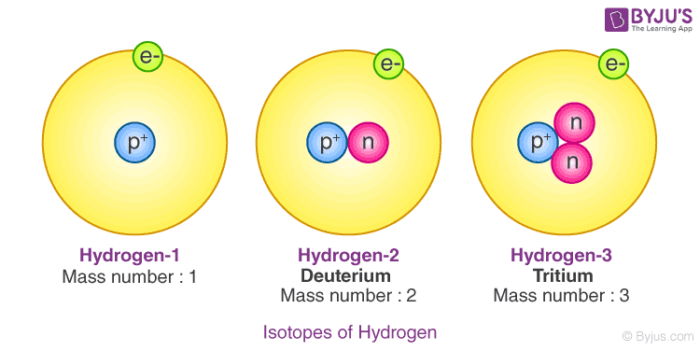
The 3 isotopes of hydrogen are protium deuterium and tritium. Chemical properties depend on their electronic configurationAs isotopes of an element have similar electronic configuration therefore they show similar chemical properties. It has one proton and one electron.
What are the three isotopes of hydrogen. Hydrogen 2 Deuterium has one neutron. They all contain one proton and one electron but different number of neutrons in the nucleus.
Protium 1 H 1 2. The key difference between deuterium and tritium is that the deuterium nucleus has one neutron whereas tritium nucleus has two neutrons. What is the difference between Hydrogen and Deuterium.
They have the same number of protons but have the different number of neutrons of each of the atom. Isotopes are chemically alike and differ in their physical properties. Write the formulae of the compounds of hydrogen formed with sodium and chlorine.
Tritium is a stable radioactive isotope or radioisotope of hydrogen and it has a half-life of about 123 years. How many protons neutrons and electrons are present in each isotope. Write 3 isotopes of hydrogen why do isotopes show similer chemical properties.
Hydrogen has three isotopes. STRUCTURE OF THE ATOM 21 Activity 116 2. Isotopes are atoms with the same number of protons but that have a different number of neutrons.
Again Hydrogen is an exception. Uranium isotope 235 is therefore known as Uranium235 or U235 2. Write a note on isotopes of Uranium.
Almost all the hydrogen is Hydrogen 1. Deuterium and tritium are two isotopes of hydrogen. It s symbol is 1 1 H ii.
3 - 1 2. This isotope of hydrogen contains 1 proton 1 electron and 1 neutron. 2 - 1 1.
Furthermore the mass number of deuterium is 20135532 while the mass number of tritium is 30160492. This isotope of hydrogen contains 1 proton 1 electron and no neutrons. Its atomic mass is 201410178 and it.
Deuterium is an isotope of hydrogen. Since the atomic number is equal to the number of protons and the atomic mass is the sum of protons. Isotopes is a Greek word isos means same and tope means place.
First used by Soddy. Hyphen Notation the mass number is written with a hyphen after the name of the element. Isotopes have a different number of neutrons but they have the same number of protons and electrons.
This isotope of hydrogen contains 1. Isotopes are defined as one of two or more atoms of the chemical element with the same atomic number but different atomic mass. Isotopes are atoms of an element whose nuclei have the same atomic number but different mass number.
Compared to other hydrogen isotopes deuterium has a mass number of two one neutron and one proton in the nucleus. Hydrogen 1 Protium has no neutron. Write their atomic composition schematically and explain.
Hydrogen 2 Deuterium. Hydrogen has three isotopes ie hydrogen 1 1H 1 1 H deuterium 2 1H 1 2 H and tritium 3 1H 1 3 H with mass numbers 1 2 and 3 respectively. 4 rows Name the isotopes of hydrogen.
This is because atoms of an element can differ in the number of neutrons Q. Explain their symbolic designation using the isotopes of hydrogen. Isotopes of Hydrogen.
Protium and Deuterium are classified under the stable isotopes of hydrogen. The three isotopes of uranium are. The main difference between the three isotopes of Hydrogen are the number of neutrons in the nucleus.
Hydrogen has no neutrons Deuterium has. These individual nuclides are called isotopes of that element. Hydrogen - 1 Protium.
It is defined as. As we know that hydrogen element has three isotopes which are protium deuterium and tritium. The isotopes of hydrogen are unusual in that they have distinct names.
Tritium 3 H 1 or T The mass ratio of protium deuterium and tritium is 123. Isotopes of an element have the same atomic number number of protons but different atomic mass numbers due to different number of neutrons in their nuclei. Deuterium 2 H 1 or D and 3.
Hydrogen has three isotopes. 234 92 U 235 92 U and 238 92 U. It is defined as the element that have the same number of protons but have the different number of neutrons of each of the atom.
Among the other heavier isotopes of hydrogen 5H is the most stable and 7H is the minimum stable.

The Three Isotopes Of Hydrogen Video Lesson Transcript Study Com

The Three Isotopes Of Hydrogen Video Lesson Transcript Study Com

The Three Isotopes Of Hydrogen Video Lesson Transcript Study Com
Belum ada Komentar untuk "Write the Isotopes of Hydrogen Explain Their Differences"
Posting Komentar